Voucher Specimens & Collecting Spiders
One of the great ironies of biology is that biologists spend a fair amount of their time killing the organisms that they study. Don’t get me wrong, I’m not saying that collecting doesn’t have it’s place, but we must recognize it for what it is. Having said this, I should also note that most biologists avoid destructive collection whenever the objectives of their studies can be met in other ways; or if the populations of the organisms in question are vulnerable.
In the case of spider study, collecting is still relatively frequent. There are two reasons for this. First, with the exception of a few large or distinctive species, most spiders cannot be identified without microscopic examination. Serious study of an organism usually begins with identification. Second, because of their relatively small size and high reproductive capacity, most spiders have high populations in suitable habitat. There are frequently hundreds to thousands of individuals per hectare. For those species, removal of a few individuals is unlikely to adversely affect the health of the population. Above all, think before you collect. Is it really necessary to kill the specimens? If so, are you prepared to take the basic data and label the specimens so that they are useful as permanent records?
Permits
The Ohio Division of Wildlife has no general permit requirements for collecting spiders in Ohio. The Division requires that the collector obtain permission (preferably in writing) from the landowner before collecting. When collecting on public land this would involve contacting the public agency responsible for the area. This is particularly important if you may be collecting in ODNR natural areas, nature preserves, or Ohio State Parks. Some of these areas are closed to collecting and others will require a specific permit.
Fluids for Preserving Spider Specimens
The standard preservative is 70% ethyl alcohol (grain alcohol, ethanol). This is not easy to obtain and is often expensive. The best substitute is 70% isopropyl alcohol. This is usually fairly easy to obtain at a local pharmacy or drug store, sold as “rubbing” alcohol. It is usually sold pre-mixed to 70%. Some spider collectors use 80% alcohol in the field because the water in the bodies of the spiders will dilute the solution somewhat, and because it kills the spiders a bit faster. If you choose to do this, just transfer the spiders to 70% alcohol when you return home. Do not use denatured alcohol as the denaturing agent may have unpredictable effects on the long-term stability of the specimens and may make them useless for DNA analysis. Another fluid to avoid is “ethyl rubbing alcohol.” This is denatured with other solvents such as xylene, toluene, or acetone that will damage the spider specimens.
Data and Labels
The most critical portion of any sort of study is the collection of accurate data. To ensure that the spider visual record or voucher specimen is to be of most value it is crucial that information about the record be made in written form. The best way to do this is to write it down immediately. Memory is imperfect and often incomplete. If you write down the particulars of where a spider was found, including the situation as well as location, there is a greater chance that you will provide accurate and complete data. For spider specimens, this means putting a label directly into the vial with the spider. Labels glued or tied to the outside of the container inevitably are lost or inadvertently exchanged with other labels. Labels in the fluid with the spider are much safer. Obviously the label must be relatively small to fit in a vial, but with care a clear label can be written on a small slip of durable paper or a piece cut from a note card. Most professional biologists use permanent-ink pens for labels, these can be obtained from art or office supply stores as India ink or permanent ink pens. One good choice are Pigma Pens. Alcohol will dissolve most inks, so you need to test the ink in alcohol. Ball-point pen inks are almost always unsuitable. The most inexpensive method is to write in pencil. This is permanent in alcohol and will not fade with time.
Some workers print labels for commonly-visited localities. This is an excellent method provided that the printing is permanent. Sadly, most computer printer inks are water or alcohol soluble. Laser printed and photocopy labels will work in the short term but after ten years or so the lettering may fall off the paper. There is nothing like the experience of picking up a vial with a valued specimen and noticing that the label has dissolved or that the lettering has separated and lies like black alphabet soup at the bottom of the vial! I have found that Lexmark Black#17 ink for my inkjet printer is permanent in alcohol (for at least 11 years).
The label itself must include the exact locality and date that the spider was collected. Additional information about the technique used and the particular situation can be written on the back of the label. You may also wish to include a field number that refers to more extensive notes which you may record in a field notebook. If you do this you should provide a copy of these field notes at the time of contribution of the specimen to a museum collection.
Sample label (front)
Making the capture
The easiest way to capture and collect spiders is to scare them into a dry container (such as the empty plastic container) and then transfer them into a container with alcohol. Alternately the plastic container can be placed in a freezer for a few minutes. In the freezer the spider will enter torpor and die relatively quickly and may experience less trauma. Carbon dioxide gas can also be used to anesthetize spiders before collecting. The following are a few basic methods used when collecting spiders.
- Visual search: Walk through the habitat and search visually for spiders, their webs or retreats (curled leaves, silken cases). Walk or crawl low through the habitat checking under loose bark, fallen wood, debris, rocks etc. Always remember to return the fallen logs or rocks to the same position where you found them. These micro habitats can be very important to small animals, so it makes sense to minimize your disturbance impact. Walls of houses, buildings and especially basements are also excellent spider hunting grounds. This method can be especially interesting at night because a completely different fauna emerges after dark.
- Sweep net: Using a heavy insect net (or even a pillowcase stretched over a wire clothes hanger) sweep through the top foot of loose soft vegetation or tall grass. Don’t try this where there are berry vines or roses because the net will snag and the spiders will escape. Remember to use quite a bit of vigor as you sweep or the spiders may fall off the vegetation in front of your net. After a few (dozen?) sweeps, dump the contents of the net onto a flat sheet and capture the spiders as they run away. Be prepared to act quickly! Sort carefully through the remaining material, you may find a number of spiders curled up and “playing possum” among the debris. It is not uncommon for a sweep sample to capture a dozen species representing four to seven families after a few minutes of work. This is one of the best methods of capturing active hunters such as jumping, lynx, nursery-web, sac or ghost spiders. Small web-building species are also frequently captured.
- Beating sheet: This method is much like sweeping. In this case spread the cloth sheet under a bush or the low branches of a tree. Grab the branches and give them a vigorous shaking, alternately strike them with a stick or stiff branch. Spiders (and other creatures) will be dislodged and will fall onto the sheet. As before, be ready because they will move quickly to hide. Some spiders may return to the branch up a silk drag line that they left when they fell.
NOTE: Beating and Sweeping do not work well in wet conditions. If there is a heavy dew or if it has rained recently the net and the spiders will stick to the wet cloth and are often damaged or killed during the sweeping. They are much less likely to tumble into the net. Avoid sweeping in such conditions.
- Pitfall Trapping. One of the most effective methods of capturing ground-living spiders is pitfall trapping. Any smooth-sided container buried flush with the ground surface will work. Some people prefer to have a funnel at the top of the container and the plastic “disposable” cone-shaped plastic cups can be modified for this purpose. Inside the pit you may want to put a second cup so that you can remove the contents without disturbing the edge of the pit. This edge is probably the most crucial key to success. If a spider detects a lip or a ridge, she is likely to walk around rather than tumble into the trap. Rain can be a problem so you may wish to place a cover over the pit propped up about an inch or so above the edge. The smaller the gap between trap and lid will reduce accidental captures of small vertebrates (toads etc.). Sometimes pits are left “dry” but most collectors use ordinary auto antifreeze in the pits. This will kill and preserve the captives with minimal evaporation. Both methods have potential problems. If you leave pits dry you may find only one large (well-fed) spider or centipede, smaller ones will have been killed and/or eaten. If you use wet pits the fluid may attract wildlife which could be poisoned. Antifreeze is available in two forms: ethylene glycol-based and propylene glycol-based. Propylene glycol is preferred because it is not as toxic. If you do use a toxic liquid in your pits you must cover the pits with wire screen or other barriers to keep wildlife out. If you put out pit traps you should be prepared to check them frequently. Some nocturnal raiders may dig up and destroy your pits. Raccoons, skunks and opossums are infamous for this behavior. To prevent this, cut a piece of “chicken wire” or “hardware cloth” screening about 2 feet square. Place over your pit and pin down the four corners with tent stakes or heavy bent wires. The mesh is wide enough that small creatures (such as spiders) will pass through undisturbed and the edge is usually far enough from the trap that it will discourage inquisitive raccoons from attempting to tunnel into the trap. Remember to remove the pit and re-fill your hole when you are finished collecting!
- Litter Sampling. Using gloves, collect a large amount of leaf litter in a black plastic trash bag or similar container. Be sure to include the leaves and duff at the surface of the soil as this will have many small animals. Keep the bag of litter cool until you are ready to extract the spiders. A litter sample left in the sun will bake and the dead spiders will be impossible to find. Our standard sample unit is 1 m2 area of ground.
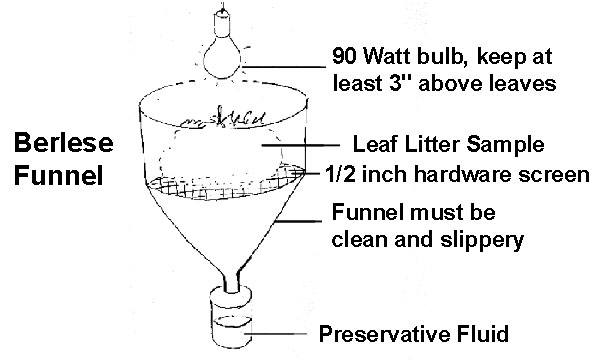
- Berlese Funnel method: place the sample over a large funnel that has been fitted with a 1/4-1/2 inch hardware-cloth or wire screen. The funnel should be placed in a rack with the opening over a cup with alcohol. Suspend a bright light over the top of the leaf litter (not too close, leaves burn!). In the laboratory we use a 90W bulb but always ensure that the bulb is at least 3 inches away from the top of the litter. If you use a 25W or 40W bulb you can place the bulb a bit closer but never let it touch the leaves. As the pile of leaves dries out the small organisms will migrate down and eventually fall through the funnel into the alcohol-filled cup. Crude illustration of a Berlese Funnel is shown below: